PCOS Part 1: Pathophysiology of PCOS
Table of Contents
Pathophysiology of PCOS
Diagnostic Criteria
Insulin Resistance and Leptin Resistance
Authors
Many of us don’t realize how well the intricate system of feedback loops in our reproductive endocrine system work until they are disrupted in some way. Polycystic ovarian syndrome (PCOS) represents an example of this. It is a disorder characterized by a collection of symptoms, and is prevalent in patients who present at infertility clinics, affecting 5-10% of women at reproductive age. An estimated 90% of anovulatory cases are related to PCOS. In addition to negatively affecting metabolic parameters and ovulation, it is also associated with several mental health issues (such as depression and anxiety) in the women who have it. In part 1, we will discuss the pathophysiology of PCOS, its diagnostic criteria, and insulin and leptin resistance. In part 2, we will address management of PCOS, psychological implications, and the clinician’s unique role in supporting the patient with PCOS.
Pathophysiology of PCOS
In ovulatory women, under the influence of a properly functioning hypothalamic-pituitary-ovarian (HPO) axis, the menstrual cycle is characterized by the growth and development of (usually) a single follicle that is extracted from that month’s cohort (group of follicles). In response to GnRH stimulation, the anterior pituitary gland secretes two important gonadotropins: Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH). FSH acts on the ovary to help grow and mature small follicles. That month’s dominant follicle is one which has acquired the most FSH receptors. This follicle will continue to grow and mature at the expense of the remaining small follicles, which then get reabsorbed by the body (but are still deducted from the woman’s total egg supply). Growth of the dominant follicle generates estradiol production and elevated estrogen levels signal FSH production to cease via a negative feedback system, but a high and sustained estrogen level will trigger a one-time surge of LH which causes ovulation to occur.
In a woman with PCOS the HPO axis does not express normal functionality. The pulsatile hormone GnRH is altered, resulting in increased LH activity by the pituitary gland. This increase in LH increases theca cell stimulation (see Fig 1), which produces androstenedione and testosterone, two androgens, and the resulting hyperandrogenic milieu of the ovary precludes normal follicular growth, maturation and ovulation. The ovary, then, becomes comprised of many small, antral follicles that never become dominant. The collection of these follicles can cause an increase in the size of the ovaries and generate a slightly elevated basal serum estrogen level. It remains unknown why PCOS occurs and whom it affects, but it is thought that genetics and environmental factors have a complex interplay in its emergence and clinical manifestations.
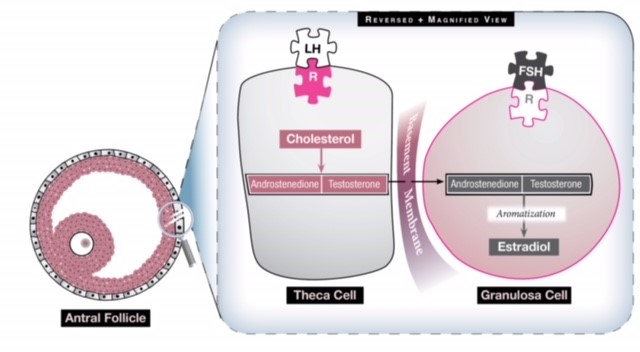
Fig 1: There are two cells in the ovary that contribute to estrogen production and they work synergistically. The theca cell converts cholesterol to two androgens (androstenedione and testosterone) under the influence of LH. These androgens then travel through the basement membrane into the granulosa cell which, under the influence of FSH, converts them to estrogen via a process called aromatization. Excess LH stimulation, then, will generate more androgens than can be converted to estrogen, so the estrogen level never becomes elevated enough to generate an LH surge and the ovary has a hyperandrogenic milieu.
Diagnostic Criteria
PCOS is not defined or diagnosed by one simple symptom and is often a diagnosis of exclusion for women who have oligo-ovulation and evidence of hyperandrogenism (such as acne alopecia and hirsutism (male-pattern hair growth and texture) once other disorders are excluded. It affects women of all shapes, sizes, and backgrounds. Although symptoms can start at menarche, most clinicians are reluctant to diagnose a relatively newly menstruating adolescent with PCOS as menstrual cycle irregularity is normal in the first year post menarche and can resolve in time. The diagnostic criteria most commonly used today were revised in an international expert workshop in Rotterdam, The Netherlands, in 2003 and are called The Rotterdam Criteria where the following were established: PCOS can only be diagnosed when a patient has at least two out of three features: oligo/anovulation, hyperandrogenism (biochemical or clinical), and the appearance of polycystic ovaries upon ultrasound. Hyperandrogenism is diagnosed either clinically (by the clinician observing androgenic symptoms) or biochemically (such as elevated serum free testosterone levels).
These criteria were revised in 2018 by an international committee which made a few changes. First, due to the availability of sensitive transvaginal ultrasound machines, polycystic ovarian morphology (PCOM) is characterized by the presence of 20 or more follicles (<10 mm) in either ovary or a ovarian volume ≥ 10 ml on either ovary as seen by transvaginal ultrasound, often situated around the periphery of the ovary (or ovaries). The 2018 guidelines also state that if a woman has irregular menstrual cycles and hyperandrogenism that the ultrasound is not necessary for diagnosis, although many clinicians still prefer to perform this. Anti-Mullerian Hormone (AMH) levels are often elevated in PCOS patients, although this is not specific to PCOS as elevated levels can be found in women without the condition. In PCOS-affected women, an elevated AMH level is reflective of a higher number of follicles arrested in the pre-antral and antral stages that fail to ovulate.
Other conditions that can cause irregular menstrual cycles (pregnancy, hypo- and hyperthyroidism, ovarian failure and hyperprolactinemia) and hyperandrogenism (congenital adrenal hyperplasia, adrenal tumor and androgen-secreting tumor) must be ruled out first, so in addition to serum bHCG levels, basal FSH and LH levels, thyroid stimulating hormone (TSH), prolactin, total and free testosterone, 17 hydroxyprogesterone (17OHP), dehydroepiandrosterone sulfate (DHEAS) are drawn. One of the most difficult differential diagnoses is discerning a woman with functional hypothalamic amenorrhea (FHA) versus a lean woman with PCOS. Classically women with FHA have a low BMI, but it also can be in the low/normal range. Both conditions are characterized by anovulation and ovaries which appear to have many small follicles in a resting state. While hyperandrogenism is not a component of FHA, women with the condition may have hirsutism due to their ethnicity, further confusing the clinical picture. One way to distinguish FHA from PCOS is with blood testing and ultrasound examination. Women with FHA often have low to normal basal FSH and LH levels (due to hypo-stimulation of the ovaries) and a low estrogen level whereas women with PCOS typically have elevated serum LH levels and low to normal FSH levels. On ultrasound, the uterus and ovaries of women with FHA are small or small/normal, whereas women with PCOS typically have an increased ovarian volume (>10 ml). There is emerging research on a possible connection between both FHA and PCOS as not all women present with characteristic features of either condition and FHA and PCOS do have some overlapping characteristics.
Insulin Resistance and Leptin Resistance
Although the diagnosis of insulin resistance (IR) is not part of the Rotterdam Criteria, it is highly prevalent in women with PCOS. An elevated BMI increases the chance that a woman with PCOS has IR, but even non-obese women with PCOS are far more likely than their size-matched counterparts without PCOS to develop insulin resistance. In addition to the health consequences of IR (such as metabolic syndrome and type 2 diabetes mellitus), it also exacerbates and contributes to hyperandrogenism in a patient population who is already suffering from it.
The gold standard for diagnosing insulin resistance is to use a hyperinsulinemic euglycemic clamp, a test which must be performed in a hospital setting. To most, this is unreasonable, so indirect testing for IR is done. In women with PCOS in a preconception clinical setting, the suggestion is to do perform an oral glucose tolerance test (OGTT) given the high risk of women with PCOS to develop impaired glucose tolerance and gestational diabetes in pregnancy. Although somewhat time-consuming, this test is preferred over fasting plasma glucose and insulin levels alone as it can diagnose impaired glucose tolerance at an earlier stage. In women with PCOS who are not in a high-risk category (i.e., BMI<25 kg/m2, not trying to conceive, no personal or family history of impaired glucose tolerance) obtaining at least baseline fasting glucose, insulin and hemoglobin A1c levels can be helpful in order to get a ‘snapshot’ of that patient’s glycemic status.
When a woman has PCOS, being overweight or obese intensifies the metabolic consequences. White fat cells are metabolically active. At a normal level, they are protective as they provide a safe home for lipids and keep fat out of organs. When there are too many fat cells, they can get overloaded and burst, releasing fatty acids into the bloodstream which can affect every organ. These fat cells get ‘stuck’ between the cells in organs and cause them to be stiff, damaged, less functional and cause chronic inflammation. It is not uncommon to diagnose ‘fatty liver’ in a woman with PCOS who is obese, as the liver is particularly vulnerable. In addition, an excess in adiposity can perpetuate existing hyperinsulinemia and insulin resistance by disrupting the delicate balance of cytokines and hormones produced by adipose tissue (see Figure 2), for example, decreasing the production of cytokines which increase insulin sensitivity, and increasing those which promote inflammation and insulin resistance. Excess insulin further contributes to abdominal adiposity and hyperandrogenism creating a vicious cycle in PCOS patients that can be difficult to overcome.
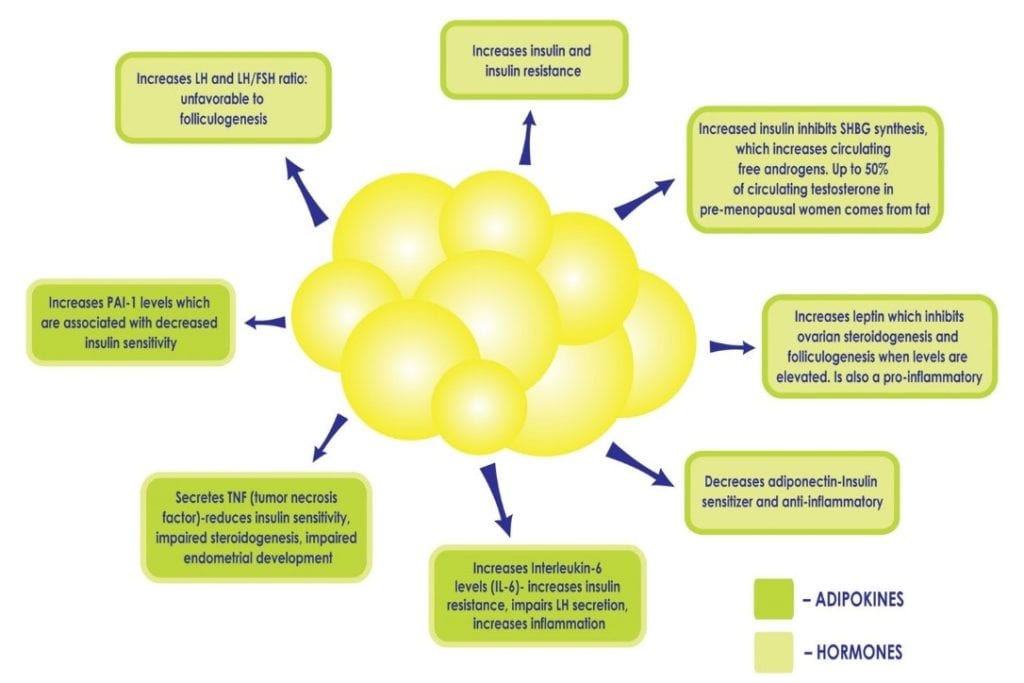
Figure 2: Increased adiposity, and the subsequent increase in fat cells, can perpetuate hyperinsulinemia and insulin resistance by disrupting the delicate balance of hormones produced by adipose tissue, such as decreasing the production of adiponectin, a cytokine which increases insulin sensitivity and increasing others which promote inflammation.
In addition to insulin resistance, patients with PCOS and obesity may also suffer from what some term leptin resistance. Some studies have shown that leptin levels are higher in obese PCOS patients compared to lean patients. Leptin is a protein produced by adipose tissue which regulates the body’s energy balance and appetite. When properly functioning, an increase in leptin signals the brain to reduce a person’s appetite and a decrease in leptin does the opposite, it signals the brain to increase appetite to provide the fuel needed for energy. In many PCOS patients with obesity, however, this system is faulty and, despite increased leptin concentrations secondary to the increase in adipose tissue, the efficacy of leptin decreases, leading to leptin resistance. Leptin resistance is considered an important risk factor for the pathogenesis of overweight and obesity, as the body remains insensitive to elevated levels and signals to the woman that she is still hungry/not satiated even after eating. Many women with PCOS complain of ‘never feeling full’ due to this resistance and continue to eat, leading to an increase in adipose tissue, which results in increased leptin resistance and perpetuates this damaging cycle. As a result, the conditions of overweight and obesity are common in women with PCOS and weight loss can feel impossible since intuitive eating is not effective when hunger and satiety cues are unreliable.
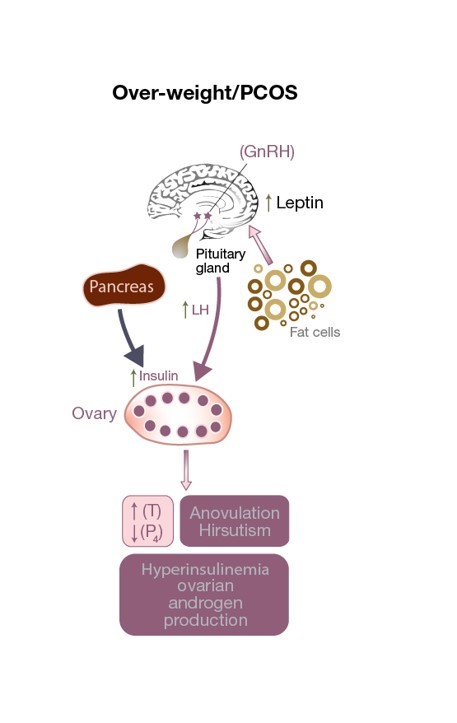
Figure 3: Disruption of the HPO axis in a PCOS patient. There is excess LH stimulation on the theca cell resulting in an increase in testosterone levels, an androgenic ovarian environment, and anovulation (resulting in low progesterone levels). Increased leptin levels due to an excess of adipose cells affect GnRH secretion. Elevated insulin levels contribute to hyperandrogenism.
Impaired leptin secretion not only affects body weight but can have a detrimental effect on ovulation (see Fig 3) and even fertilization in normal-weight PCOS patients. It alters the release of GnRH from the hypothalamus, decreasing anterior pituitary stimulation (and therefore FSH and LH secretion), and preventing the development of a mature oocyte. In addition, the granulosa cells also store and produce leptin, and high levels of leptin decrease their aromatization capacity which ultimately interferes with the ability of a dominant follicle to produce adequate amounts of estrogen (see Fig 1). A small, observational study found a direct correlation between the concentration of leptin found in the follicular fluid (FF-leptin) (which has been correlated with fertilization rate) in lean women with PCOS who have underwent IVF when compared to normally ovulating, weight-matched women.
Authors
Jaclyn Carr, BSN
Brianna Giannotte, BSN
Special thanks to Neil Chappell, MD for his assistance with editing this article.
You can visit the Fertility Nurse Newsletter website here
Want to be notified about upcoming Fertility Newsletter posts here at The ObG Project?
Get the ObG Insider e-Newsletter »Are you an
ObG Insider?
Get specially curated clinical summaries delivered to your inbox every week for free




